Noble Gas Configuration Chart
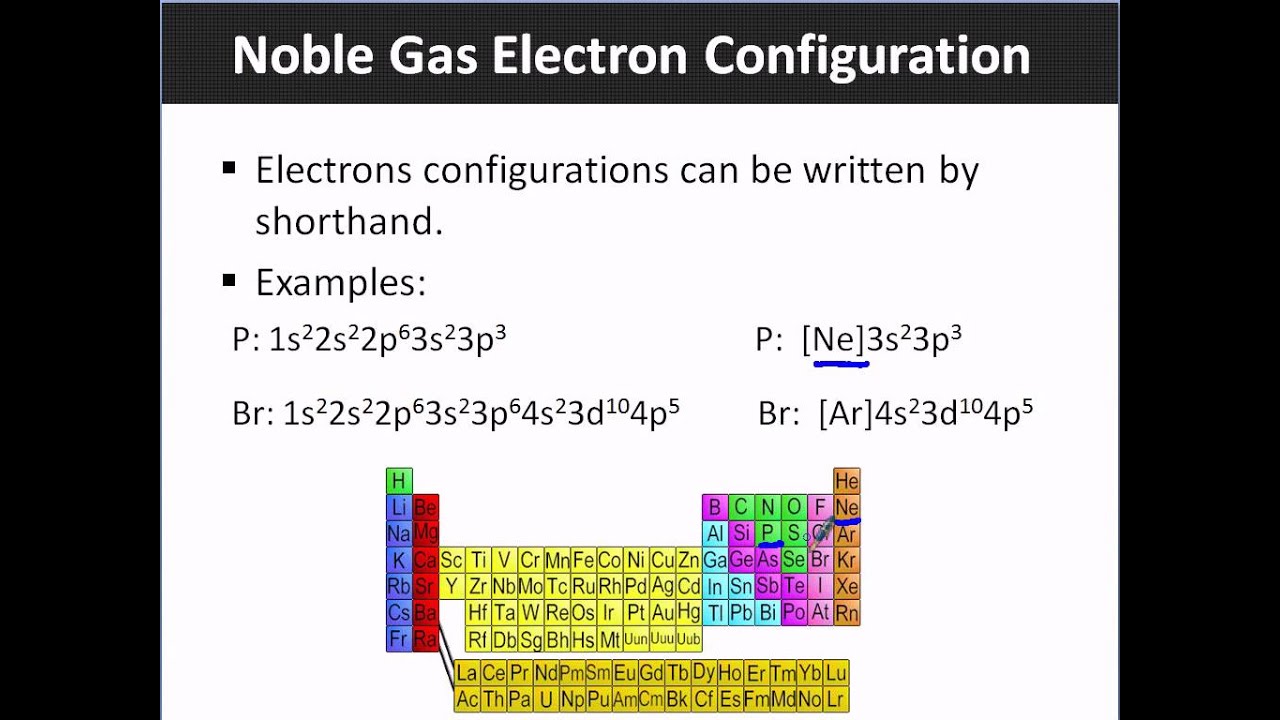
Sodium noble gas configuration becomes Left Ne Right 3S 1.
Noble gas configuration chart. Follow the steps below to write short cut version of electron configurations. Start by drawing its orbital notation for the outermost valence electrons. Electron configuration chart noble gas notation.
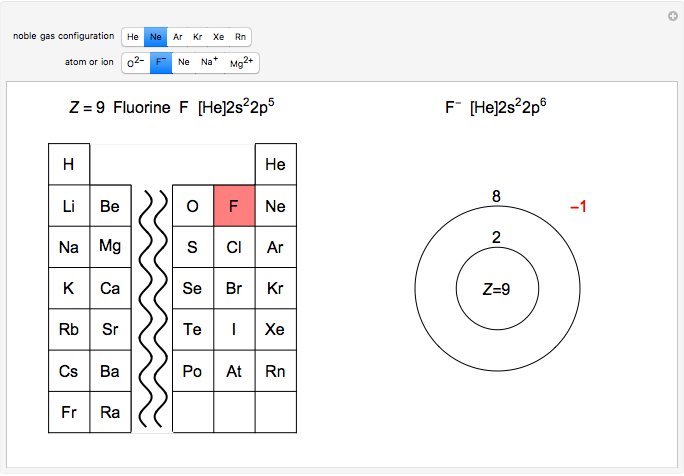
The distribution of electrons in respective atomic orbits of the noble gases is called noble gas configuration. Embed this widget. For example calcium is element 20.
81 Characteristics of Many -Electron Atoms 82 The Quantum-Mechanical Model and the Periodic Table. In order to achieve noble gas configuration to become stable it requires one electron then it will acquire the configuration of neon noble gas. Electron Configuration and Chemical Periodicity.
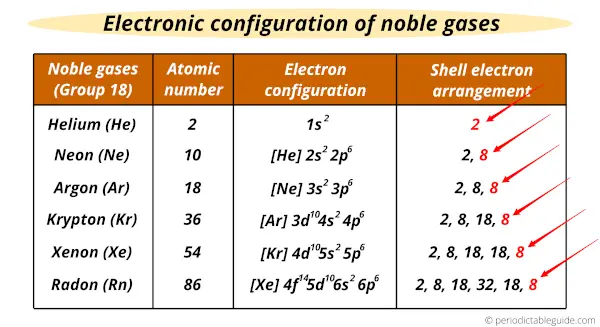
Below is a list of the noble gases and their periods. Therefore valency of chlorine is1. Group-18 elements are mostly chemically unreactive because of their stable electronic configuration and thus are known as noble gases or inert gases.
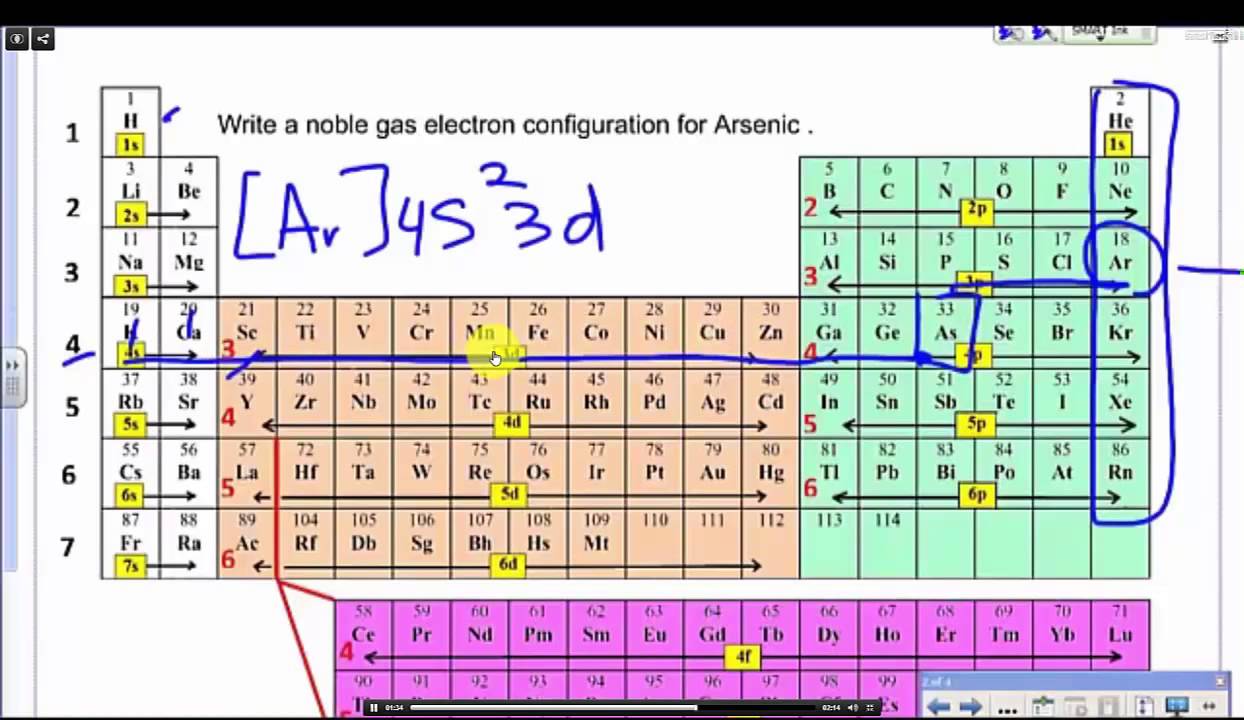
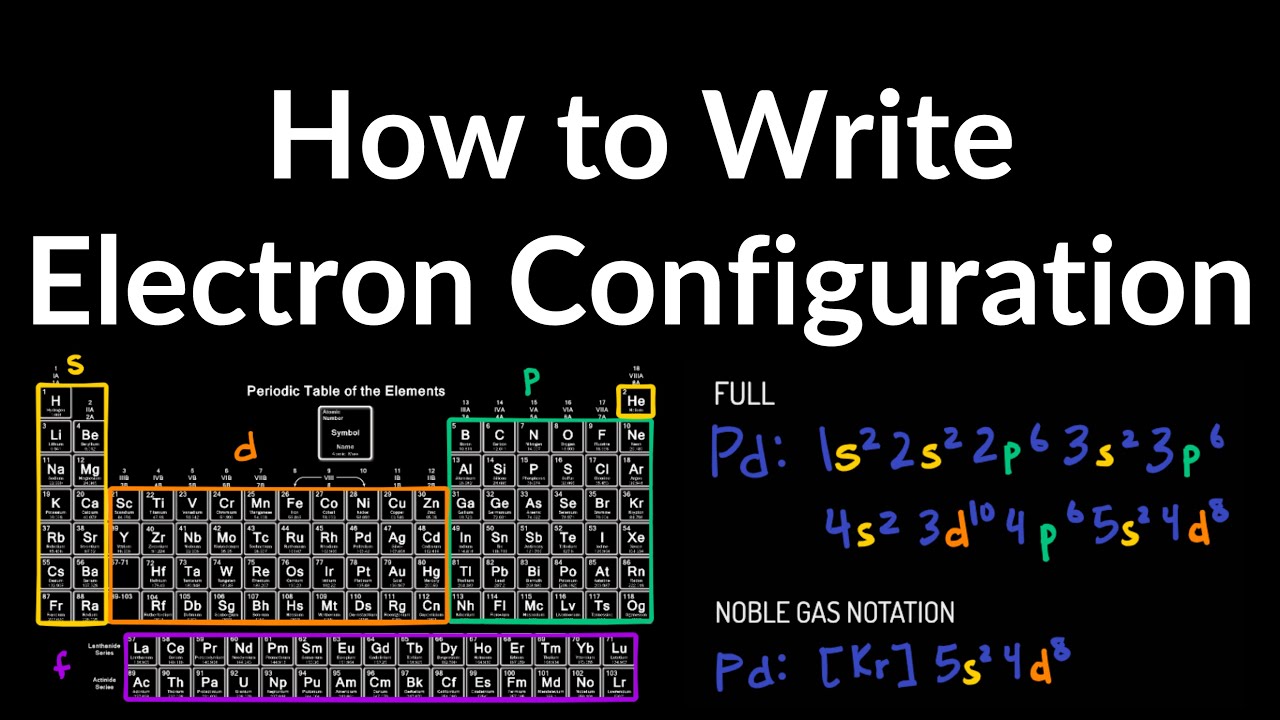
Now find the atomic number of the first noble gas in the periodic table. 107 rows The common shorthand notation is to refer to the noble gas core. Write the noble gas symbol in parentheses to start its electronic configuration.
2 10 18 36 54 and 86 respectively. This would make the electron configuration for copper 1s22s22p63s23p64s23d9 or in noble gas configuration Ar 4s23d9. 119 rows Electron configuration of Boron B He 2s 2 2p 1.
Under standard conditions they are all odorless colorless monatomic gases with very low chemical reactivity. Find the element on the periodic table. Rn7s1 If you recognize where each of the blocks of the periodic table are and that each period has a shell number n you can figure out electron configurations pretty quickly just by looking at the position of the element on the.
The basis of any reaction taking place between chemicals is due to their impulsive behavior of achieving a complete electronic configuration and reaching a level of stability. Write the symbol of the noble gas in brackets to start your electron configuration. A noble gas configuration of an atom consists of the elementary symbol of the last noble gas before that atom followed by the configuration of the remaining electrons.
83 Trends in Three Atomic Properties 84 Atomic Properties and Chemical Reactivity. Copper i number of valence electrons. 119 rows ELECTRON CONFIGURATION.
What is the electron configuration chart. 1s2 2s2 2p6 This represents 2 electrons in the s subshell of the first energy level 2 electrons in the s subshell of the second energy level and 6 electrons in the p subshell of the second energy level. Go back to the last noble gas that was passed atomic number.
1s 2 2s 2 2p 1. The prime examples are the noble gases He Ne Ar Kr Xe and Rn containing one of the magic numbers of electrons. The six naturally occurring noble gases are helium neon argon krypton xenon and the radioactive radon.
How to Find Electron Configuration. Enter an elements name or symbol to see its noble gas electron configuration. LinkSelectrons Atomic structure and subflows ã Electron Configurations and periodic Tablea writing Electron Configuration Box and Arrow Configurations using Pauli Exclusion Principle and Hund Ruleã As numbers writing Electron Configuration Sublevel Configuration- Electron distribution throughout the Sublevelsbohr.
Their general electronic configuration is of the type inert gas ns2np6 except for Helium whose configuration is 1s2. These gases are colorless odorless and chemically inert although a few compounds of Kr Xe and Rn have been synthesized in recent years. Noble Gas Electron Configuration.
The noble gas you will use will be located in period three. Put the atomic number of the noble gas under this symbol so that you know the number of electrons. A For K Z 19 condensed configuration.
Thus for sodium we replace the replacement of ne Right for the 1s 2 2s 2 2p 6 part of the configuration. That means the every element tries attain stability by acquiring noble gas configuration for which it tries to either gain electron or donate electron. Added Aug 1 2010 by ddoughan in Chemistry.
Oganesson is a synthetically produced highly radioactive element. Noble gas except those in filled inner sublevels. The noble gases make up a class of chemical elements with similar properties.
Send feedback Visit WolframAlpha. Chloride ionThe chloride ion now has the electron configuration of the noble gas argon 1s22s22p63s23p6. What is 1s2 2s2 2p6.
8-31 Sample Problem 82. Its electron configuration of 2-8-19-1 allows it to work well as a pure element and in a variety of compounds.